The purpose of developing Standard Operating Procedure (SOP) of the Research Committee at Sri Manakula Vinayagar Medical College and Hospital is to give a clear idea to undergraduate/post-graduate/faculty researchers about its proposal processing pathway. The Research Committee at Sri Manakula Vinayagar Medical College and Hospital consists of faculties from pre-clinical, Para-clinical and clinical disciplines. The Research Committee aims to offer timely and complete critical appraisal to the submitted research proposals and offer technical guidance to those who submit their proposals for its review. The review of the submitted research proposals is an in-house exercise, where an attempt is made to assess its feasibility, to improve relevance to the local context, technical quality and ethical aspects of proposed research. We encourage Good Clinical Practice and Good Authorship Practices at Sri Manakula Vinayagar Medical College and Hospital. Hence, SOP is developed with a following objective –
To ensure that the proposed research has relevance in the present context and that it is technically sound and meets ethical standards.
In order to achieve the above mentioned objective, following activities are done to –
Introduction
The proposal should have an “Introduction’ section which states the ‘need’ for the present study. It should have a brief note on what is known to the science on the given topic and what ‘new’ will be added by doing the present study. It should state as how this study is going to benefit the current state of practice/medical care/education etc.
A brief review of literature
It should include some known facts and some existing gaps in the knowledge. It is better to review the recent articles from the indexed journals. An attempt should be made to know as what is happening at international level, national level and regional level. It should also explore the strengths and limitations in the previously reported studies.
Material and Methods
In the ‘Methods’ section, please define the setting (Laboratory/hospital/community/college) in which the present study will be done. Also, specify under which Department the proposed study will be done.
Study design
Please specify the study design
Study participants/subjects
Human/Animals/Laboratory samples/Secondary data
Sample size
In quantitative research, sample size should be worked out on the basis of a ‘primary outcome’ of the study and justified. It is better to avoid feasible sample/convenient sample in quantitative research as it affects its external validity. In qualitative research, type of sample and sampling should be worked out and described.
Sampling procedure
Once the sample size is decided, then that sample should be selected from a suitable ‘sampling frame’ by using some random selection methods, where every study participant has equal probability of getting into the study. Sampling procedures and the study period should be defined. In case of clinical trials, the details related to ‘Phase’ of the trial, randomization and blinding should be given.
Measurement
Develop a tool which is reliable and valid i.e. It measures what you want to measure more accurately. Follow standard questionnaire development practices. Please check copyright/permission issues if you are using a standard questionnaire. The details of study participants such as age, gender etc. should be mentioned.
Ethical issues
Please mention the ethical issues you are expected to face and your strategy to minimize any potential harm. Please follow guidelines on Good Clinical Practice (GCP) while conducting clinical trials and CPCSEA guidelines in the conduct of animal experiments. The consent forms for research on human subjects should have an informed consent form as per given template (see Annexure 2). Please follow Consolidated Criteria for Reporting Qualitative Research for conducting and reporting qualitative researches (Tong A, 2007). We encourage researchers to anticipate ethical issues in the proposed research and try to address it in its design and data collection.
Analysis
The details of the study variables to be measured and the appropriate statistics (test of significance, level of significance) should be given. Analysis plan should be clearly worked out at the time of proposal development. Please mention the name of statistical software to be used for analysis of proposed study data. Please consult and acknowledge the biostatistician or epidemiologist during the phase of proposal development.
Analysis
The details of the study variables to be measured and the appropriate statistics (test of significance, level of significance) should be given. Analysis plan should be clearly worked out at the time of proposal development. Please mention the name of statistical software to be used for analysis of proposed study data. Please consult and acknowledge the biostatistician or epidemiologist during the phase of proposal development.
How to submit the research proposals?
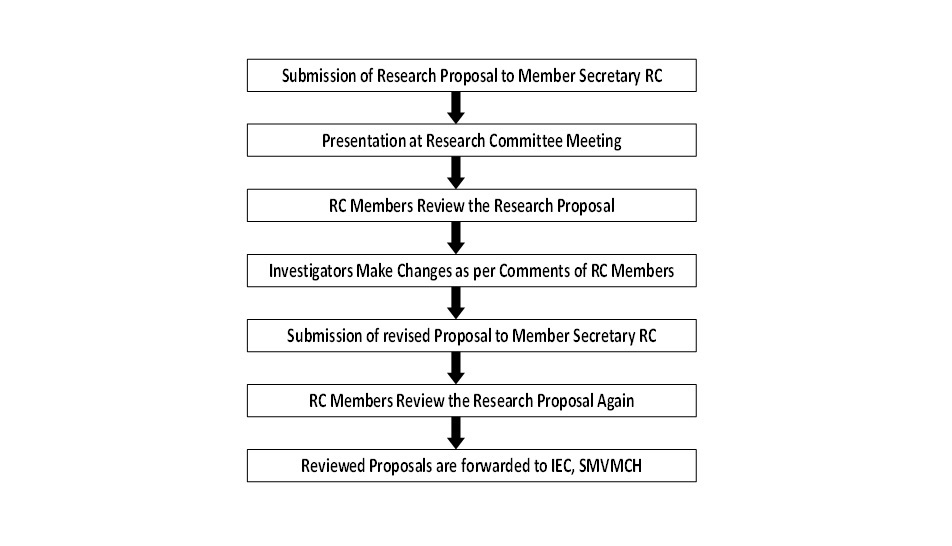
Presentation of proposal at RC committee meeting
Investigators are invited to present their proposed research work at a scheduled RC meeting. Investigators are advised to make a PowerPoint presentation of not more than seven minutes as per the template prescribed by the RC. It is mailed to the concerned presenters once they submit a soft copy to the above mentioned email ID. There will be three minutes time for questions and clarifications. The RC members will receive the soft copies of all research proposals on their respective email IDs. In the RC meeting, the members will have to review the proposals as per review template given for the research on humans (Annexure 5) and animals (Annexure 6).
There are separate review templates for research based on humans and animals. Once the presentation of the Investigator is over, the members can write their comments in the respective review templates. The members may obtain these templates from the RC member secretary quite before the RC meeting if they wish to finish the review before attending the meeting.
Comments by the RC members
Investigators are encouraged to note down the comments of the RC members during the presentation. However, all the presenters will receive the complied comments in a written communication within a week after the presentation at RC meeting is over.
How to submit the revised proposals?
Investigators have to revise their proposals in the light of comments given by the RC members. Apart from this, investigators have to respond to each comment point wise as per the given response template (Annexure 7) and make the corresponding changes in the proposal.
Fig-1:Processing of Research Proposal by Research Committee, SMVMCH
Policy for Research Scholarships at SMVMCH, Pondicherry
Purpose of the Research Funding
The purpose of research scholarships is to promote research leading to publication of original research papers by students and faculties in indexed scientific bio-medical/health journals. However, we encourage students, post-graduates and faculties to apply for funding at various regional, national and international level.
Research by the undergraduate students
Research by the postgraduate
Research by the teaching Faculties
Decision-making body
The sub-committee for research at SMVMCH will recommend the shortlisted projects for funding approval by Management of SMVMCH. Acknowledgement for funding should be mentioned in all the publications. Refer to Annexure 8 for application to seek funding.
Authorship Guidelines for Researchers at SMVMCH, Pondicherry
The reason for this authorship guideline is to –
Offer technical information and promote good authorship practices among researchers at SMVMCH and avoid duplication of efforts.
It is based on the recommendations of the International Committee of Journal Editors, Journal of American Medical Association and FAIMER guideline for authorship.
International Committee of Medical Journal Editors (ICMJE), also known as Vancouver group, 2001, states that – Authorship credit should be based on:
Conditions 1, 2, 3 must all be met. Acquisition of funding, the collection of data, or general supervision of the research group, by themselves, do not justify authorship.
To reduce the incidence of authorship problem, we encourage following tips for all the researchers at SMVMCH (COPE report, 2003).
Authorship disputes – how to handle it?
The team should have a written authorship agreement before the article is written. We recommend authors to follow authorship criteria followed by The Journal of American Medical Association, July 2007 to decide authorship. We also recommend to follow a rubric provided by Francois Cilliers, 2007 to decide the order of co-authorship. Disagreement about authorship can be classified into two types: those that do not break ICMJE guidelines (disputes) and those that do (misconduct).
(a) Disputes – It is a question of interpretation. In this case, you may discuss with the people involved and the supervisor/any senior person involved in the study/Head of the Department. Consider or support your discussion or opinion with the evidences such as laboratory notebooks, manuscripts, ICMJE statement, Instructions to Authors etc. If you remain unhappy with the supervisor’s decision, you may approach to the Research coordinator of the Research Committee at SMVMCH. But you should do this in exceptional circumstances only and other researchers are well informed about what you’re intending to do.
(b) Misconduct – If you notice or experience some scientific misconduct, please bring it to the notice of the Research Committee of SMVMCH.
Authorship Criteria
Rubric provided by Francois Cilliers, 2007
Authorship of any work resulting from this research will be determined by means of the following authorship index.
Co-authorship scoring system: Each coauthor (s) completes a self-assessment form indicating individual contribution and is encouragedto complete a form for each member of the team.
Whoever achieves a total of 25 points will be offered joint authorship in rank order of total score. In the event of ties, recent near misses will be considered. If none exists, alphabetical order may be used.
Note for authorship in case report: In the past, it was acceptable to include as authors those contributing to the management of the patient, but this is no longer true. Currently, it is expected that the authors contribute significantly to the intellectual content of the case report. Keep in mind that the best case report abstracts are those that make a small number of teaching points (even just one) in clear and succinct language. (American College of Physician, Personal Communication with Dr Gitanjali, JIPMER, Pondicherry 2013)
Criteria for Authorship used by the Journal of American Medical Association, July 2007
Each author should meet all criteria below (A, B, C, D) and should indicate general and specific contributions by checking the appropriate boxes.
Acknowledgement
We are grateful to the faculty members of the research committee for giving their valuable comments to improve these guidelines. We are especially thankful to Dr. Gitanjali, Professor in Pharmacology JIPMER, Pondicherry and Shital Bhandari, Patan Academy of Medical Sciences, Nepal for their critical review and inputs.